Hemostasis
Hemostasis is the process of blood clotting, which is required to stop bleeding at an injury site and initiate wound healing. It constitutes a fine-tuned cooperation of many blood components, including platelets, protein von Willebrand factor (VWF), and fibrinogen. In particular, VWF protein is essential in primary hemostasis, as it mediates platelet adhesion to vessel walls at high shear rates.
Formation of von Willebrand factor-platelet aggregates
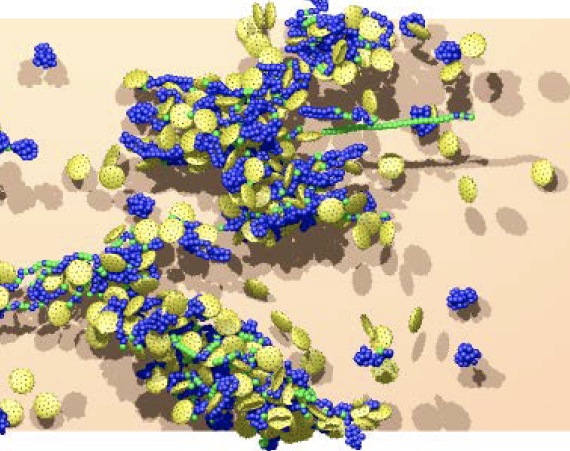
Platelets form aggregates as they adhere to stretched von Willebrand factors (VWFs) at high shear rates. The formation of VWF-platelet aggregates is reversible as they fall apart at low shear rates. In blood flow, VWF-platelet aggregates generally form near vessel walls where the shear rates are highest. When the aggregates become large enough, they migrate away from the vessel walls toward the flow bulk where shear stresses can significantly drop down. Therefore, spontaneously formed VWF-platelet aggregates would generally dissociate in the bulk of blood flow. Certain mutations of VWF may result in irreversible VWF-platelet aggregates, leading to undesired thrombotic events.
Behavior of von Willebrand factor in flow
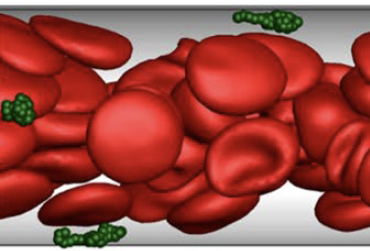
von Willebrand factor (VWF) retains its compact (globule-like) shape in equilibrium due to internal molecular associations, but is able to stretch when a high enough shear stress is applied. VWF stretching is associated with its activation, such that it is able to bind efficiently to platelets and damaged vessel walls. Mesoscopic numerical simulations demonstrate that the compact shape of VWF is important for its migration (or margination) toward vessel walls and that VWF stretches primarily in a near-wall region in blood flow, making its adhesion possible. This indicates that VWF is a highly optimized protein in terms of its size and internal associations which are necessary to achieve its vital function.
- M. Hoore, K. Rack, D. A. Fedosov, and G. Gompper, "Flow-induced adhesion of shear-activated polymers to a substrate", Journal of Physics: Condensed Matter, 30, 064001, 2018.
- K. Rack, V. Huck, M. Hoore, D. A. Fedosov, S. W. Schneider, and G. Gompper, "Margination and stretching of von Willebrand factor in the blood stream enable adhesion", Scientific Reports, 7, 14278, 2017.
- B. Huisman, M. Hoore, G. Gompper, and D. A. Fedosov, "Modeling the cleavage of von Willebrand factor by ADAMTS13 protease in shear flow", Medical Engineering & Physics, 48, 14-22, 2017.