Cell mechanics
Mechanical properties of cells are tightly coupled with their biological functions. Therefore, cell mechanics significantly affects cellular behavior and determines mechanical properties of supra-cellular structures such as tissues. We develop mechanical models of cells, which allow us to quantify experimental measurements and help us understand how different cellular structures contribute to the mechanical response and behavior of biological cells.
Cell blebbing
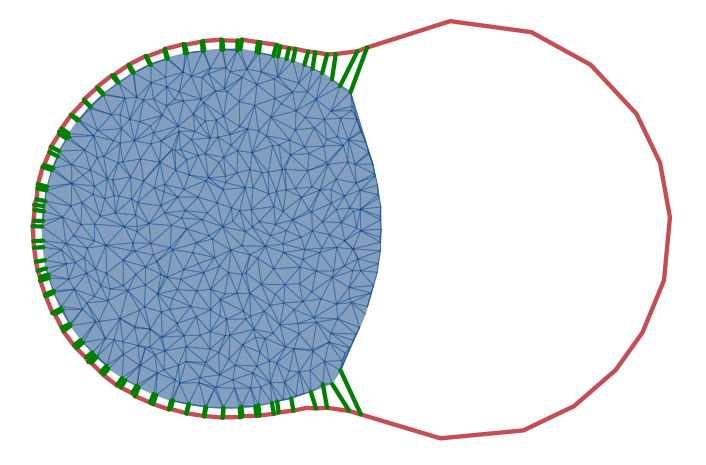
Cell blebbing corresponds to the dissociation of cell membrane from the inner cellular network as result of internal stresses. Therefore, it represents the instability of the connection between the membrane and actin cortex. We employ a coarse-grained cell model to study cell blebbing for a number of involved parameters, including membrane rigidity, cytoskeletal stress, and binding strength between the membrane and bulk cytoskeleton. Furthermore, theoretical model for the detachment of bound solid surfaces is extended in order to include the effect of deformable cellular structures on the blebbing process.
Membrane fluctuations
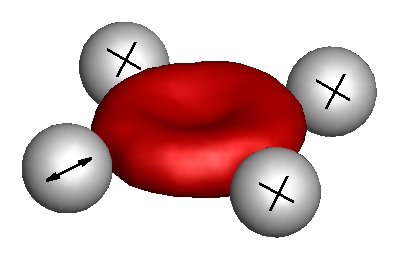
Red blood cells are seen to flicker under optical microscopy, a phenomenon initially described as thermal fluctuations of the cell membrane. However, recent studies indicate that the membrane flickering can be shape and position dependent, and can have a contribution from non-equilibrium (active) processes. We employ realistic stochastic simulations of red blood cells to investigate the dependence of membrane fluctuations on the position of measurements and to decouple passive (thermal) and active contributions to the observed flickering. Simulations indicate that it should be possible to quantitatively extract red blood cell membrane properties, including shear elasticity, bending rigidity, and membrane viscosity. We also discuss possibilities for the decoupling of passive and active contributions to the membrane flickering.
- H. Turlier, D. A. Fedosov, B. Audoly, T. Auth, N. S. Gov, C. Sykes, J.-F. Joanny, G. Gompper, and T. Betz, "Equilibrium physics breakdown reveals the active nature of red blood cell flickering", Nature Physics, 12, 513-519, 2016.
Modeling cell deformation
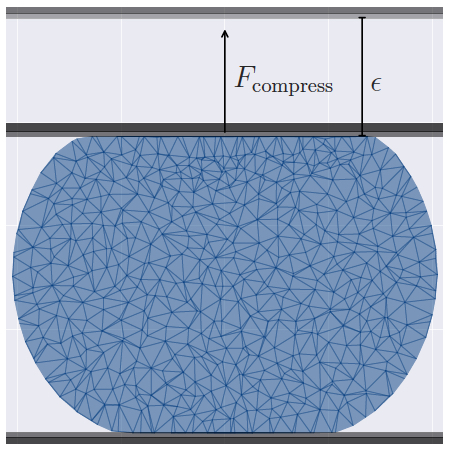
Mechanical properties of biological cells are often measured by direct cell deformation, e.g. by using optical tweezers or microplate compression as shown in the plot. However, quantitative interpretation of cell deformation is significantly complicated by the composite structure of biological cells, since they consist of a lipid membrane with an elastic cortex, bulk cytoskeleton, organelles. Ideally, we have to be able to dissect the different contributions of cell properties to the observed deformation, including membrane elasticity and bending rigidity, cytoskeleton elasticity, and internal viscosity. Using compound cell models, which represent multiple cellular components, we investigate how different cellular structures contribute to overall cell deformation.
Mechanical properties of erythrocytes
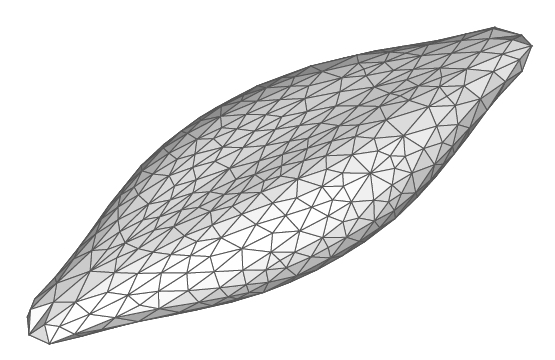
Red blood cells (RBCs) have highly deformable viscoelastic membranes exhibiting complex rheological response and rich hydrodynamic behavior governed by special elastic and bending properties and by the external/internal fluid and membrane viscosities. We have developed a multiscale RBC model which is able to predict RBC mechanics, rheology, and dynamics in agreement with experiments. The RBC linear and nonlinear elastic deformations match those obtained in optical-tweezers experiments. The rheological properties of the membrane are compared with those obtained in optical magnetic twisting cytometry. Our findings clearly indicate that a purely elastic model for the membrane cannot accurately represent the RBC's rheological properties, and therefore accurate modeling of a viscoelastic membrane is necessary.
- D. A. Fedosov, B. Caswell, and G. E. Karniadakis, "A multiscale red blood cell model with accurate mechanics, rheology, and dynamics", Biophysical Journal, 98, 2215-2225, 2010.
- D. A. Fedosov, B. Caswell, and G. E. Karniadakis, "Systematic coarse-graining of spectrin-level red blood cell models", Computer Methods in Applied Mechanics and Engineering, 199, 1937-1948, 2010.