Blood flow
Blood is a physiological fluid that circulates in our cardiovascular system and consists of red blood cells (RBCs), white blood cells (WBCs), and platelets suspended in plasma. The cardiovascular system is a closed circuit which can be divided into three major sections: the heart, large vessels, and the microcirculation. The main function of the heart and large vessels is to maintain continuous blood flow within the vessel network, while blood flow in the microcirculation plays a fundamental role in a wide range of physiological processes and pathologies in the organism including transport of molecules and cells, organism defense, haemostasis, and various diseases.
Blood flow in idealized vessels
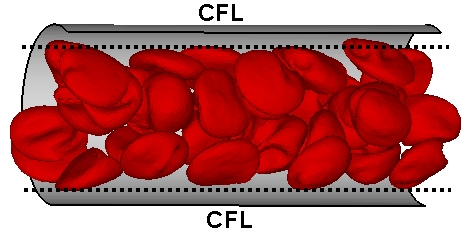
Cylindrical microchannels serve as idealized microvessels. RBCs in microvessels migrate away from the walls, due to hydrodynamic interactions between the cells and the walls, leading to a layer near a wall void of RBCs. This layer is called cell-free layer (CFL) or RBC-free layer. The CFL thickness is directly associated with the blood flow resistance such that a larger CFL leads to a lower resistance of blood flow.
We are interested in the flow resistance, migration and deformation of RBCs, and their interactions in flow. The properties of blood flow and the behavior of single cells depend on several conditions including flow rate, hematocrit (or volume fraction of RBCs), and RBC mechanical properties. In addition, interactions between different RBCs are important, since they are able to aggregate (i.e. they possess attractive interactions) into structures called "rouleaux", which resemble stacks of coins.
- D. A. Fedosov, H. Noguchi, and G. Gompper, "Multiscale modeling of blood flow: from single cells to blood rheology", Biomechanics and Modeling in Mechanobiology, 13, 239-258, 2014.
- H. Lei, D. A. Fedosov, B. Caswell, and G. E. Karniadakis, "Blood flow in small tubes: quantifying the transition to the non-continuum regime", Journal of Fluid Mechanics, 722, 214-239, 2013.
- D. A. Fedosov, B. Caswell, A. S. Popel, and G. E. Karniadakis, "Blood flow and cell-free layer in microvessels", Microcirculation, 17, 615-628, 2010.
Migration and dispersion of red blood cells
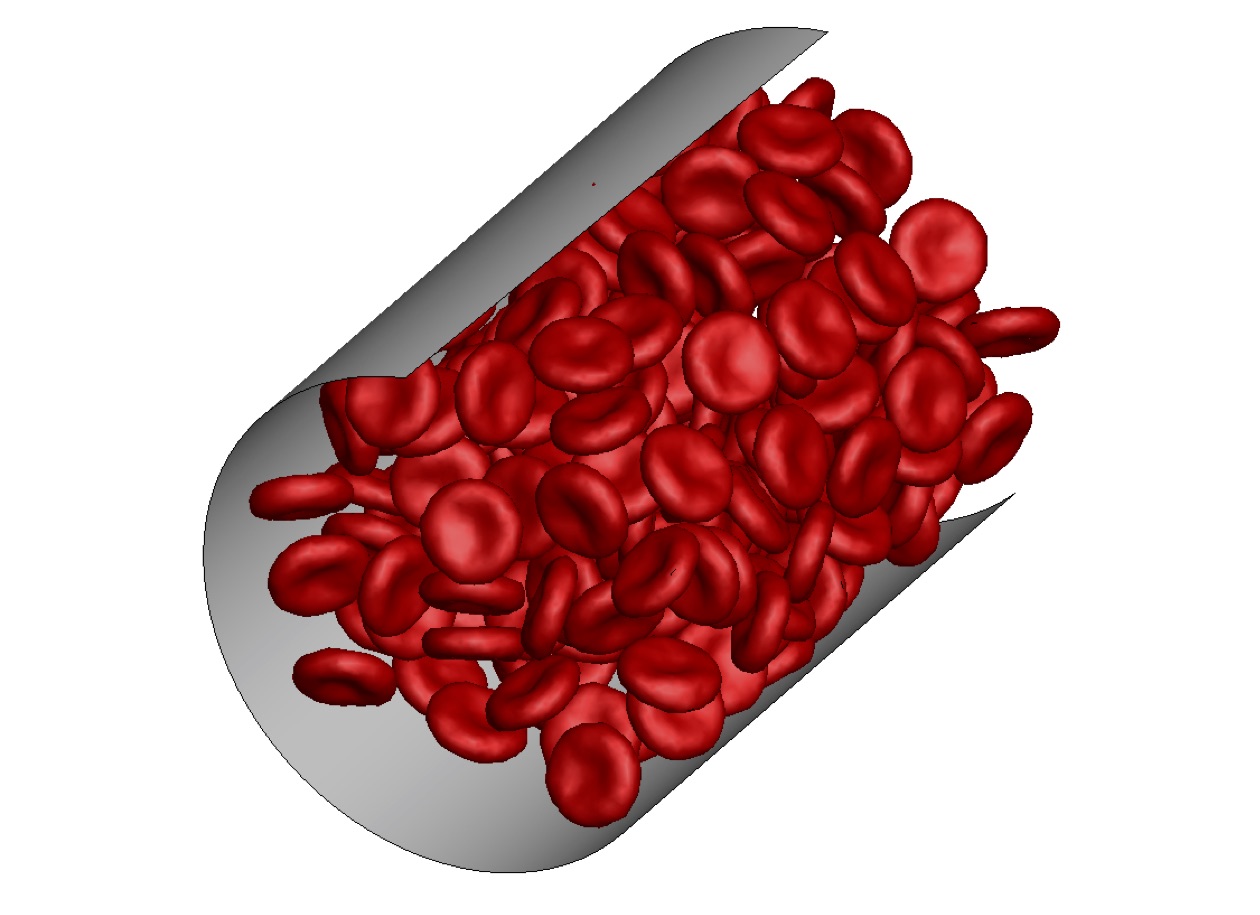
The flow resistance in microvasculature strongly depends on the distribution of red blood cells across vessel cross-sections, which may be significantly distorted at vessel bifurcations and junctions. Therefore, we have investigated the development of blood flow and its resistance starting from a dispersed configuration of red blood cells for different conditions. Initially dispersed red blood cells migrate toward the vessel center leading to the formation of a cell-free layer near the wall and to a decrease of the flow resistance. The development of cell-free layer appears to be nearly universal when scaled with a characteristic shear rate of the flow. The universality allows an estimation of the length of a vessel required for full flow development, l ~ 25D, for vessel diameters in the range 10 μm < D < 100 μm. Thus, the potential effect of red blood cell dispersion at vessel bifurcations and junctions on the flow resistance may be significant in vessels which are shorter or comparable to the length l.
- D. Katanov, G. Gompper, and D. A. Fedosov, "Microvascular blood flow resistance: role of red blood cell migration and dispersion", Microvascular Research, 99, 57-66, 2015.
Blood flow in microvascular networks
The microcirculation (or microvascular network) is comprised of the smallest vessels (e.g. arterioles, capillaries, venules) with diameters up to about 100 μm. Microcirculatory vessels provide the routes for the circulating blood. Typically, the microcirculation geometry resembles a tree-like structure following the route of blood passing through arterioles, capillaries, and venules. Blood flow in microcirculation is extremely complex and diverse with dramatic changes in flow rates and patterns. This complexity is attributed to non-trivial vessel geometries and blood rheological properties which depend on blood cell mechanical characteristics, aggregation interactions, local hematocrit (RBC volume fraction), and flow conditions.
We are interested in the properties of microcirculation perfusion, including blood flow and pressure distribution, hemodynamic resistance, and blood cell trafficking. In addition, the effects of the endothelium surface layer (or glycocalyx), RBC aggregation, and vessel wall elasticity on the microvascular flow characteristics need to be considered. Furthermore, we are interested in vascular drug delivery by micro- and nano-particles with an aim to characterize their trafficking and distribution in microcirculation.
- G. Gompper and D. A. Fedosov, "Modeling microcirculatory blood flow: current state and future perspectives", WIREs Systems Biology and Medicine, 8, 157-168, 2016.